Manage the Compliance Demands of the Complex EU Framework
Facilitate Continual Improvement of Device Safety and Performance
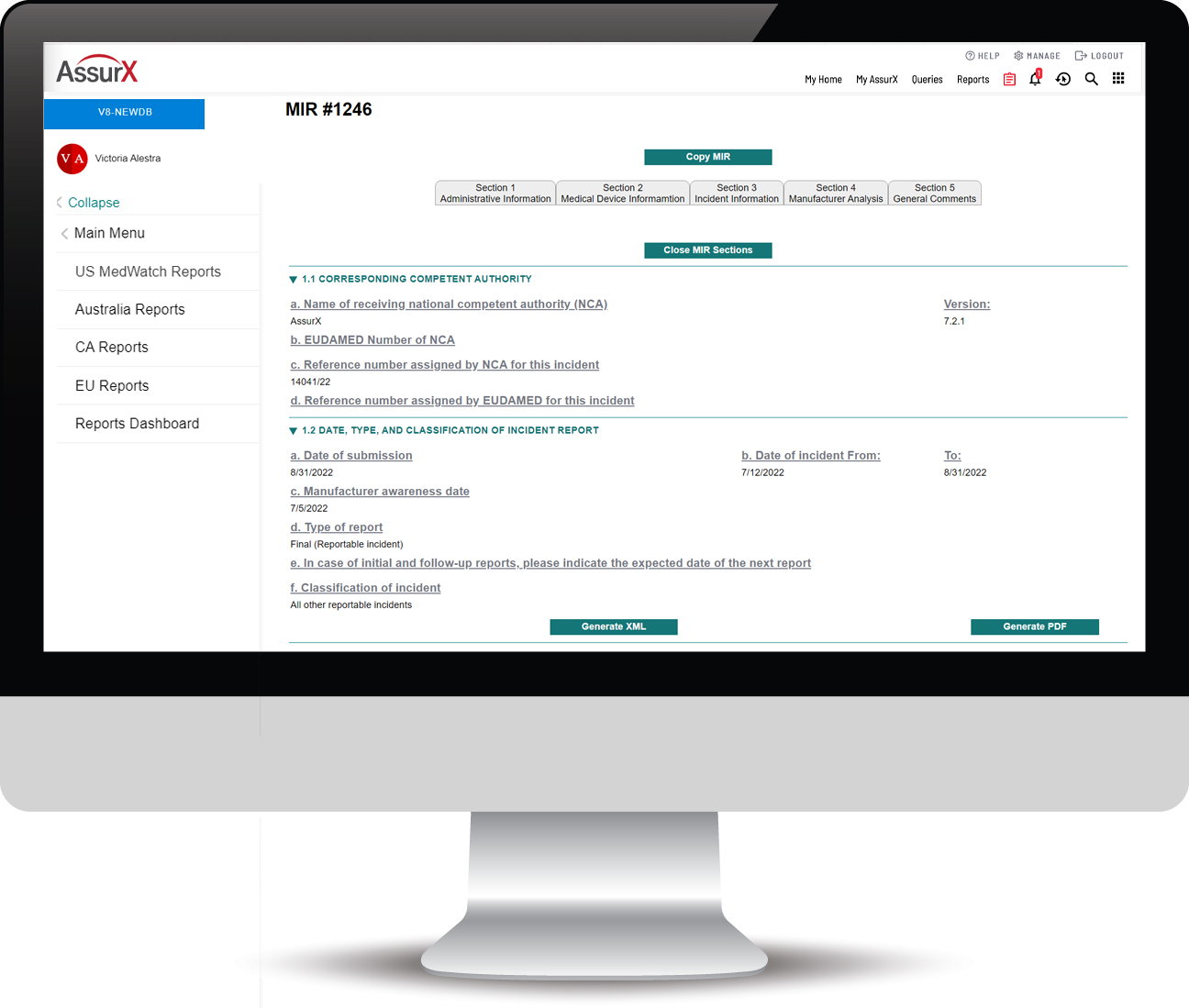
The AssurX EU MDR Solution Automates the Process of MIR Submissions
European Union Medical Device Regulation (EU MDR) requires medical device manufacturers to meet significantly higher quality and safety standards than in the previous Medical Device Directive (MDD). Post-market vigilance requirements present process challenges that require modern technology solutions.
AssurX electronic reporting solution automates the EU Manufacturer Incident Reporting (MIR), enabling timely post-market reporting of applicable medical device vigilance incidents. Post-market requirements outlined in the EU MDR carry significant process challenges and procedure updates.
The AssurX EU MDR solution aligns business logic with the most up-to-date MIR template published by the European Commission (EC) and automates the documentation and submission under vigilance guidelines and reporting time frames.
AssurX EU MDR Solution Benefits
COMPLIANCE
Submit reports within required time frames. Reminders and escalations keep users aware of deadlines, incident severity, and process the next steps: built-in audit trail and electronic signature functionality.
CENTRALIZE
Collect detailed, quantifiable data for trending in a centralized repository. Integrate with other QMS processes for downstream issue management, including investigations, CAPA, and change control.
CONTROL
Robust monitoring and reporting engine provide deep insight into device issues for investigations and continual product improvement.
STREAMLINE
Minimize risk and through centralized control of the EU medical device event reporting process.
FLEXIBILITY
AssurX allows organizations to adapt processes in alignment with changing regulatory landscapes. Quickly incorporate EU MDR reporting changes as regulation is updated.
VISIBILITY
Get real-time trending and global oversight with features that provide full visibility into the current state of incident reports.
AssurX Manufacturer Incident Reporting (MIR) submission data can be connected to any AssurX QMS process that requires the compilation and evaluation of product performance data.
AssurX Automated MIR Submission Solution Features
The AssurX EU MDR enables electronic reporting of medical device vigilance events required by manufacturers, user facilities and importers in compliance with European Union Medical Device Regulation (EU MDR) and requirements of the Manufacturing Incident Report form (MIR). The EU MDR solution can run standalone or integrated with our modern post-market surveillance quality management system for unmatched visibility into the device history.
One Platform. Every Solution.
Seamlessly integrate all quality and compliance processes with one software system. Start by using just the solutions you need now, then easily add more as you are ready. No other system is easier to deploy, configure, and modify.





