Maximize Quality Operations and Minimize Risk
Tear down quality process silos and set the foundation for continuous improvement.
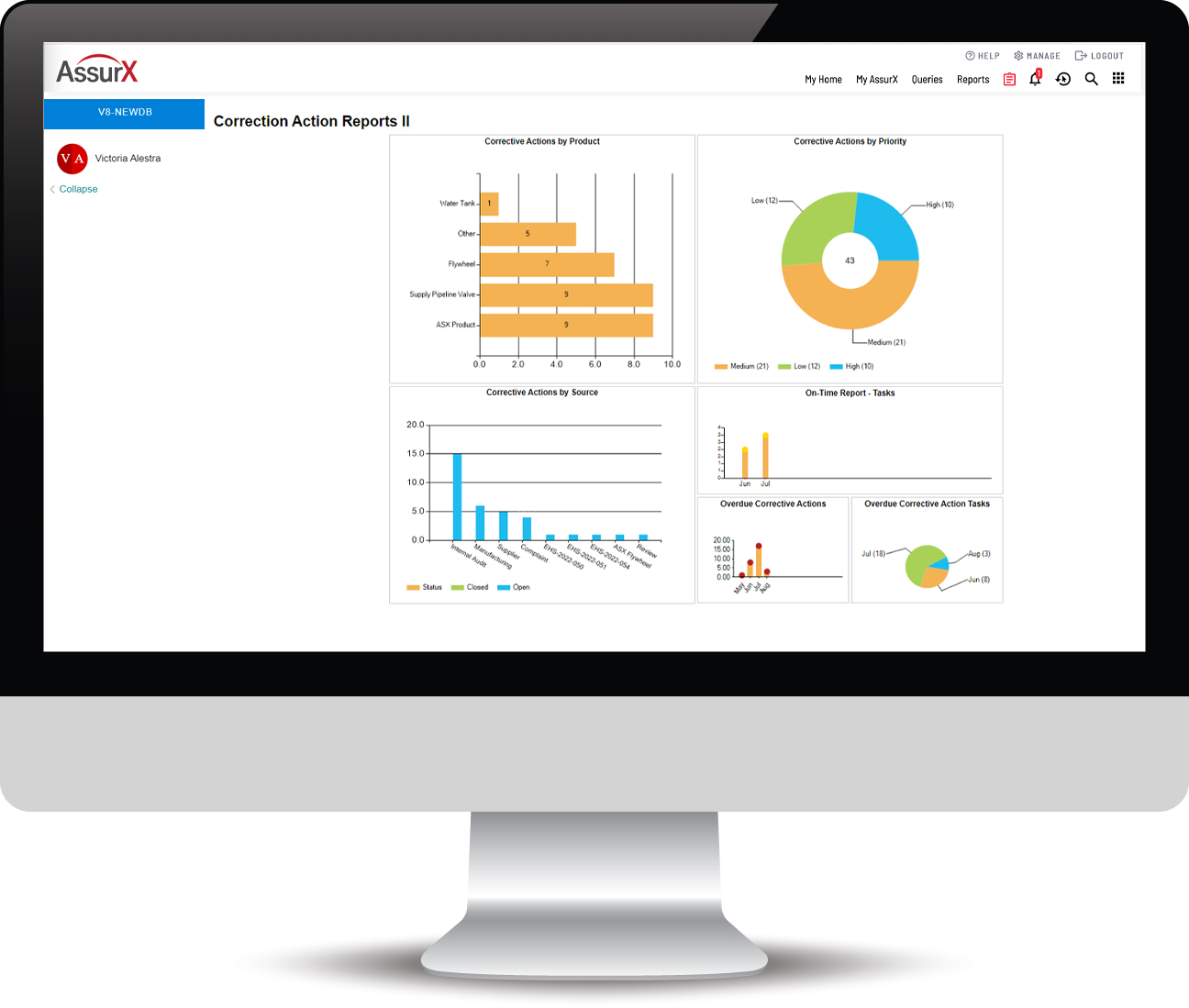
Closed-Loop CAPA Management Software
Using AssurX, you can automate all the steps required to evaluate and investigate deviations, as well as implement the appropriate actions and controls to prevent further quality incidents. AssurX CAPA management also helps you track improvements and perform thorough root cause analyses. When the corrective and preventive action (CAPA) is integrated with additional processes, data and documents, it drives quality improvements even further.
AssurX CAPA management software enables your enterprise quality management system to be self-correcting, guiding your organization from incident capture to closure while creating continual improvements in your quality chain.
AssurX is designed to address regulatory requirements in regulations such as:
CAPA Software for Continuous Quality Improvement
CAPA management software is a modern essential for regulated companies, but more importantly, it’s a basic element of continuous quality improvement. AssurX connects the CAPA process with document control, change management, training, deviation, nonconformance, audit, and other quality processes.
AssurX CAPA provides a centralized platform and robust tools that can enable your organization to:
How AssurX Modernizes Your CAPA Management Process
AssurX eliminates paper-based and unconnected electronic systems and the related costs associated with day-to-day manual operations. By automating the CAPA process and integrating with additional quality processes with the following features and benefits:
Compliance Excellence
An end-to-end approach to CAPA actions provides significant compliance advantages. If process gaps are found at any time during the CAPA process, a change record can be launched. If documentation needs to be updated, AssurX Document Management gets the revision completed and approved, and can trigger a training task.
On-Demand Data
Provides efficient data and document retrieval and management. Reporting includes the ability to display specific records, including any attached images. Always know exactly where you are in the CAPA process. Trending and data analytics provide intelligence to pinpoint related issues and take proactive measures to address them.
Integration
CAPA can be launched anywhere in your electronic quality chain. Audits, nonconformances, deviations, supplier quality issues, and customer complaints all connect in a trackable, closed loop. With AssurX, you’ll be able to clearly determine that you have a quality issue and seamlessly trigger a CAPA.
Data Maximization
AssurX integrates with other business applications to provide greater insight and collaboration (for example, in CAPA investigations). Connect with MES, LIMS, ERP, CRM, and others to integrate your applications under a single platform to engage with other business areas.
Centralization
Automate all CAPA-related tasks (routing, follow-up, escalation), under a centralized system to eliminate process vacuums.
Efficiency
AssurX CAPA management software speeds your time to CAPA completion. Tracking, automated reminders, escalations, and configurable dashboards keep assigned users on-path to task closure.
Auditability
AssurX captures all related CAPA details including signatures and documentation, creating a controlled way to organize report findings and attach related evidence records in one place.
Control Risk
A risk-based approach facilitates identification, analysis, and prioritization of risks. As a result, the appropriate actions can be taken to proactively control and minimize the chances of future negative outcomes.
One Platform. Every Solution.
Seamlessly integrate all quality and compliance processes with one software system. Start by using just the solutions you need now, then easily add more as you are ready. No other system is easier to deploy, configure, and modify.




